Green chemistry research yields a safer method for synthesizing fluoride complexes

Gaby Clark
scientific editor

Robert Egan
associate editor

Chemical synthesis lies at the heart of modern science and technology, enabling the creation of various pharmaceuticals, agrochemicals, and functional materials. While the demand for chemical synthesis grows with scientific advancements, it comes with the costs of environmental pollution and hazardous waste. To combat the same, researchers are now turning towards sustainable alternatives using green chemistry approaches.
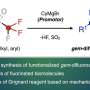
One such chemical process which is in urgent need for greener alternatives is fluorination. Fluorine-based organic compounds find applications in a variety of industries, ranging from pharmaceuticals to electronics. These compounds are synthesized through the process of fluorination using different fluorinating agents like potassium fluoride (KF) and quaternary ammonium fluorides like tetrabutylammonium fluoride (Bu4NF).
While these reagents are promising, their reactivity is often hindered due to low solubility (as in the case of KF) and high hygroscopicity (seen in Bu4NF). This calls for the development of novel fluorinating agents with suitable properties and better reactivity.
Against this backdrop, a team of researchers led by Professor Toshiki Tajima from Shibaura Institute of Technology, Japan, came up with an exciting solution. The team developed a new fluorinating quaternary ammonium complex by combining KF with tetrabutylammonium bromide (Bu4NBr). The newly formed quaternary ammonium tri(1,1,1,3,3,3-hexafluoroisopropanol)-coordinated fluoride (Bu4NF(HFIP)3) showed extremely low hygroscopicity and was found to be an excellent fluorinating reagent for electrochemical fluorination. The findings are published in Chemical Communications.
"KF is a safe, affordable fluorinating agent, but its poor solubility in organic solvents has limited its use. We had been exploring ways to make it more effective," explains Prof. Tajima. "It all clicked only after we discovered it readily dissolves in HFIP."
To develop the fluorinating complex, the team started by dissolving KF in HFIP and Bu4NBr in dichloromethane, respectively. Once dissolved, both solutions were mixed together for 30 minutes and were then subjected to filtration and purification.
The resultant product was a viscous and clear liquid of Bu4NF(HFIP)3. The chemical composition of the product was confirmed through NMR spectroscopy studies. Furthermore, the approach was also applied to other quaternary ammonium bromides for the synthesis of different reagents.
The resultant products showed low hygroscopicity, which is favorable for a greater shelf life. Additionally, the synthesis only involved a basic ion exchange reaction using KF, which makes the method simpler and inexpensive. Moreover, the method is also safer compared to other synthesis methods, making it a greener alternative for fluorination.
"The new fluorinating agent we developed in this study can have a range of applications in the synthesis of pharmaceuticals, agrochemicals, functional materials, molecular probes for PET inspection, and many more," remarks Prof. Tajima.
Many industries use fluorinating agents for the synthesis of organofluorine compounds. Having a safer fluorinating reagent that is easier to handle could be a game-changer and is a significant milestone in the field of green chemistry.
By overcoming the limitations of two different fluorinating reagents to form a novel fluorinating agent, the research has bridged a critical gap in the process of fluorination, opening avenues for sustainable and effective synthesis strategies.
More information: Haruka Homma et al, Facile synthesis of R4NF(HFIP)3 complexes from KF and their application to electrochemical fluorination, Chemical Communications (2025). DOI: 10.1039/D5CC01341K
Journal information: Chemical Communications
Provided by Shibaura Institute of Technology