Preventing stalling to improve CAR-T cells' efficacy against tumors

Gaby Clark
scientific editor

Robert Egan
associate editor

Chimeric antigen receptor (CAR)-T cells are a promising cancer therapy that are made from the patient's own T cells, which are reprogrammed to fight their cancer. One of the limitations of CAR-T cell therapy is the ability of these cells to survive long enough to target the entire tumor.
Once injected back into the patient, the CAR-T cells tend to rapidly expand when they become activated by the tumor cells, but eventually die off due to a natural process called activation-induced cell death.
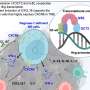
In a study published in Science Translational Medicine, a research team discovered a way to alter CAR-T cells so they can partially avoid activation-induced cell death, which allows them to live longer and better fight off the tumor.
This study was a follow-up to previously published studies where they found that INFg was necessary for CAR-T cells to kill solid tumor cells, but not blood cancers.
IFNg is a cytokine released from CAR-T cells (and normal T cells) when they become activated that induces inflammation. If too much IFNg is released, it can cause toxicities in patients. Therefore, they created CAR-T cells that did not release IFNg.
In blood cancers, this led to decreased inflammation without affecting how well the CAR-T cells kill the tumor. However, in solid tumors, CAR-T cells that did not release IFNg did not kill tumor cells as well.
In both cases, CAR-T cells not releasing IFNg tended to expand more and live longer—two characteristics that would be advantageous for CAR-T cell efficacy.
In this study, the researchers created CAR-T cells that still release IFNg (to maintain their ability to kill solid tumors) but continue to expand more and live longer, as if they are not releasing IFNg.
They used CRISPR/Cas9 to knock out expression of the IFNg receptor (IFNgR) in the CAR-T cells. Without this receptor, IFNg has no way of signaling to the CAR-T cell.
The researchers used T cells from healthy donors to make the IFNgR-knockout CAR-T cells and examined their function in response to cancer cell lines in a dish.
They also injected these CAR-T cells into mice with tumors to demonstrate their improved persistence and function in a preclinical model.
They found that knocking out IFNgR in CAR-T cells boosted their expansion, persistence and anti-tumor activity in both dishes and mouse models, enhancing their effectiveness and durability.
CAR-T cells that were unable to respond to IFNg signaling underwent less cell death following activation—i.e. deleting the IFNgR preventing the CAR-T cells from stalling.
Overall, this led to increasing CAR-T cell efficacy and expansion in multiple models of solid tumors.
These findings suggest that knocking out IFNgR from CAR-T cells would improve their efficacy for targeting any tumor type by prolonging their survival and allowing them to kill more cancer cells.
The researchers hope to initiate a clinical trial of these CAR-T cells in patients with solid tumors, either in collaboration with a company or as a spin-out endeavor.
Marcela Maus, MD, Ph.D., director of the Cellular Immunotherapy Program and the Paula J. O'Keeffe Endowed Chair of the Mass General Cancer Center, is senior author and Stefanie Bailey, Ph.D., Hana Takei, and Giulia Escobar, Ph.D. of the Krantz Family Center for Cancer Research at Massachusetts General Hospital are co-lead authors of a paper
More information: Stefanie R. Bailey et al, IFN-γ–resistant CD28 CAR T cells demonstrate increased survival, efficacy, and durability in multiple murine tumor models, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adp8166