Abstract
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal soft tissue sarcoma of the gastrointestinal tract. Correct diagnosis with thorough use of pathologic and molecular tools of GIST mutations has been of the foremost importance. GIST are usually (95 %) KIT positive and harbor frequent KIT or platelet-derived growth factor receptor α-activating mutations. This deep molecular understanding has allowed the correct classification into risk groups with implications regarding prognosis, essential use in the development of targeted therapies and even response prediction to this drugs. Treatment has been evolving and an update to include lessons learned from recent trials in advanced disease as well as controversies in the adjuvant setting that are changing daily practice, is reviewed here. An effort from the Spanish Group for Sarcoma Research with investigators from the group has been undertaken to launch this third version of the GIST guidelines and provide a practical means for the different disciplines that treat this complex disease.
Similar content being viewed by others
Prologue
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors originating in the digestive tract. They have a characteristic morphology, are generally positive for CD117 (c-kit) and are primarily caused by activating mutations in the KIT or PDGFRA [1]. On rare occasions, they occur in extravisceral locations such as the omentum, mesentery, pelvis and retroperitoneum.
GIST have become a model of multidisciplinary work in oncology: the participation of several specialities (oncologists, pathologists, surgeons, molecular biologists, radiologists…) has allowed advances in the understanding of this tumor and the consolidation of a targeted therapy, imatinib, as the first molecular treatment that is effective in solid tumors. Following the introduction of this drug, median survival of patients with advanced stage GIST has increased from 18 to more than 60 months. Additionally, sunitinib is another targeted drug registered as second-line treatment for metastatic GIST.
Diagnosis
Radiology
Radiological diagnosis of GIST is similar to that of other digestive-tract tumors. In barium studies, GIST appear as submucosal lesions [2] and in ultrasound studies as hypoechogenic masses that, when large, can displace neighboring structures and show cystic, necrotic or hemorrhagic areas.
A computerized tomography (CT) scan and magnetic resonance imaging (MRI) are the first choice to study location and extension [3]. A CT with contrast and image acquisitions of the arterial and portal phases allows identification of hypervascular hepatic lesions that would otherwise go unnoticed and become evident when they turn hypodense with treatment. The latter could wrongly suggest progression due to the development of new lesions. On the contrary, a CT scan without endovenous contrast allows detection of a hemorrhage or intratumoral calcification.
In a CT, tumors appear as well-circumscribed exoluminal masses that, after the contrast, show heterogeneous enhancement, especially large tumors, which may have necrotic-hemorrhagic areas or degenerative components [4].
An MRI is useful for the local study of tumors located in the pelvic area [5] as well as for the study of the mesenteric and peritoneal extension. With PET, small GIST have a homogeneously increased uptake, while in large lesions (>4 cm), uptake may be heterogeneous [6].
Histology
Techniques for histological diagnosis
The technique of choice for providing histological diagnosis is echoendoscopy-guided biopsy or a CT-guided percutaneous biopsy when the first option is not possible. Although FNA (fine-needle aspiration) endoscopy could be performed on esophagogastric tumors, this technique does not usually provide sufficient material to carry out a proper, definitive histological diagnosis, thus a biopsy would also be called for. If the biopsy become complex, a laparoscopic incision or laparotomy is required in order to obtain diagnosis. However, the use of biopsy forceps for polypectomy increases the risk of perforation and should be avoided and performed in exceptional cases only [7].
Preoperative endoscopic biopsy is not necessary when a lesion is considered suspicious, resectable or operable. On the other hand, it would be appropriate for patients with disseminated disease or in locally advanced cancers when considering neoadjuvant therapy [8].
Samples should be fixed in formaldehyde and not in Boiun’s solution as it may alter results in subsequent studies.
Pathological characteristics
GIST are the most common mesenchymal tumors originating in the digestive tract. They have characteristic morphological features and are generally positive for CD117 (c-kit) and have active KIT o PDGFRA mutations [1].
Macroscopic characteristics
Most common sites They are usually found in stomach (60 %), small intestine, jejunum and ileum (30 %), duodenum (5 %), rectum (2–3 %), colon (1–2 %). They are much less frequent in the esophagus (<1 %). In some cases, there is presence of disseminated tumor with unknown primary tumor and a small number of them, and the tumor originates in the omentum, mesentery and retroperitoneum [9, 10]. Metastases are typically intra-abdominal involving the peritoneum and liver; from a distance, they are odd looking and usually found on skin, bones and soft tissue.
Morphology The size of GIST is variable (up to 38 cm). Most measure around 5 cm at the time of diagnosis. They typically originate in the digestive tract and can be submucosal, intramural or subserosal. They are rarely invasive and there is often ulceration of the mucous membrane with poor prognosis [10]. Necrotic, hemorrhagic and cystic degeneration areas are usually displayed [11]. They are usually solitary, sporadic cases usually have multiple lesions [12] in familial or neurofibromatosis GIST and Carney Triad [13]. Its growth pattern is extensive (21 %) and pseudo-extensive (45 %) or infiltrative (24 %). The pathology report must always include three-dimensional tumor measurement, and the existence of quantification of necrosis and distance between lesion and margin as incomplete resection is associated with poor prognosis [14].
Microscopic characteristics
Three histological types can be distinguished according to the cellular appearance: fusiform cells (77 %), epithelioid cells (8 %) and mixed (15 %) (1). The epithelial type is more frequently observed in stomach and epiplon [15].
The number of mitosis can vary substantially, between 0 and over 150 mitoses per 50 high-power fields (hpf). Most show a low or very low mitotic index (≤5 mitosis/50 hpf). The method for counting mitoses in the most active areas, in a total of 50 hpf (corresponding to an area 10 mm2), should be standardized given its prognostic relevance. Strict criteria should be followed as pyknosis and karyorrhexis must not be overlooked. The mitotic index should be graded as follows: low ≤5/50hpf and high >5/50hpf [16].
Immunohistochemistry
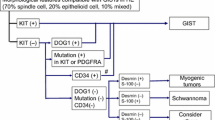
Over 95 % of GSIT have CD117 (c-kit) expression with diffuse cytoplasmic staining pattern but also rarely in the membrane or Golgi apparatus. There is intense staining in 75 % of cases. Moreover, 70–90 % also express CD34, 20–30 % actin, 8–10 % S-100 and desmin in 2–4 % [1]. IHC studies are useful in confirming a diagnosis of GIST and given the implications of diagnosis, appropriate CD117 immunohistochemistry in order to avoid errors. Staining of Ki67 is a prognostic factor and recommended [17]. DOG1 can optionally be included in the initial panel and is highly recommended in negative c-kit [18], in which DOG1 is expressed in over 35 % of cases.
Differential diagnosis
The main differential diagnosis in fusiform GIST comprises smooth muscle tumors (leiomyoma and leiomyosarcoma); schwannoma and malignant peripheral nerve sheath tumor; inflammatory myofibroblastic tumor; solitary fibrous tumor, sarcomatoid carcinoma; inflammatory fibroid polyp and desmoid fibromatosis. Differential epithelioid GIST diagnosis includes poorly differentiated carcinomas; endocrine cancers and variants of epithelioid leiomyosarcoma and malignant peripheral nerve sheath tumor. Luckily, morphological features together with an adequate immunohistochemical panel allow proper diagnosis [11].
Kit-negative GIST
Between 4 and 5 % of GIST with typical morphological features are negative for CD117 [19, 20]. Those with negative stains or weak staining <10 % of tumor extension are to be considered as such. Kit-negative GIST is clinically, pathologically and genetically different from kit-positive GIST. Although they are more frequently found in stomach, they can also be observed in the omentum and peritoneal surface. They are less commonly CD34 and actin positive, while desmin expression is approximately 30 %, especially in stomach lesions and epithelioid morphology [18]. DOG1 positive was observed in slightly over one-third of tumors [18]. Other antibodies such as PCK tetha or PDGFRA have been found not to be very reproducible or useful [21, 22].
Kit-negative GIST present a true diagnostic challenge. It is recommended to extend the immunohistochemical panel with other markers such as DOG1 and a mandatory study for KIT and PDGFRA mutation, being mindful that there is a small percentage of GIST with typical morphology, negative for CD117 and DOG1 and wild type for KIT and PDGFRA genotype [20]. Cases morphologically defined as atypical, cellular atypia, CD117 and DOG1 negative and with no KIT or PDGFRA mutations should not be classified as GIST.
Final recommendations
-
Pathologic diagnosis is based on both unique microscopic features and ancillary techniques (CD-117, CD34, actin, desmin, S-100 and ki-67), which are very important to confirm diagnosis.
-
The pathology report must include tumor size; number of mitoses per 50 HPF (10 mm2) counted in the most active regions; and margins status.
-
It is advisable to refer the complex or unusual cases to experienced centers.
-
Regarding tumors with typical morphology GIST, an extended phenotype of DOG1 as well as KIT and PDGFRA gene mutation analysis is required.
-
Albeit optional, it is convenient to include the risk group separated by site. Table 1 (Miettinen et al.) [23] and histologic grading defined exclusively by the number of mitosis (low grade ≤5/50HPF, high grade >5/50HPF).
Table 1 Primary gastrointestinal stromal tumors (GIST) risk assessment guidelines
Molecular diagnosis
GIST are characterized by activating mutations in KIT and PDGFRA genes—are shown to be mutually exclusive encoding a receptor tyrosine kinases type III (RTC) [11, 12]. KIT mutations are found in 60–85 % of GIST tumors, while PDGFRA mutations are found in 5–10 %. Approximately 10–15 % of GIST do not have detectable mutations in any of these receptors (GIST wild type), suggesting that other molecular routes can also be involved in the pathogenesis of these tumors [24–26].
Spectrum of mutations in GIST
Mutations found in GIST mainly affect exons which codify functional domains of KIT and PDGFRA receptors. Among the main types of mutation, we find the following: deletions, point mutations, duplications, insertions and complex mutations [19].
Mutation detection before tyrosine kinase (TK) inhibitor therapy such as imatinib is known as primary mutation (and mainly affects exons 11, 9, 13 and 17 of KIT, and exons 18, 12 and rarely affects 14 of PDGFRA). Meanwhile, mutations detected during treatment, which are to a large degree responsible for resistance to TK inhibitors, are known as secondary mutations (generally detected in exons 13, 14 and 17 of KIT and 18 of PDGFRA) [24, 26].
KIT mutations
The most common KIT mutations affect exon 11 (juxtamembrane domain). Approximately 70 % of GIST present some type of mutation in this exon [24, 27]. The most frequent mutations in this exon are interstitial deletions, commonly affecting the beginning of exon 11 (between codons 550 and 579) and especially codons 557–559. Then, there are point mutations, albeit with a lower incidence, and limited to four codons (557, 559, 560 and 576). Lastly, at the extreme end of the exon (between codons 571 and 591) and in a much smaller proportion of patients, we find tandem duplications associated with GIST gastric site and epithelioid or mixed cell morphology [25, 28–30].
In exon 9 (extracellular domain), only duplication of residues 502–503 have been described and is present in 9–20 % of cases depending on the study. This mutation is mainly associated with GIST of small bowel location and greater malignant potential [26, 30].
The KIT-TK domains are encoded by exons 13 and 17. Only point mutations have been found in these exons, the frequency being between 0.8 and 4.1 % for exon 13 lower than 1 % in the case of exon 17 [25, 26, 30–32].
PDGFRA mutations
Overall, the estimated frequency rate of PDGFRA mutations in GIST is 5–10 % [24, 25, 33], which are associated with localized gastric GIST and epithelioid morphology [25, 27, 33]. Mutations are concentrated in the juxtamembrane domain (0.7 %) encoded by exon 12; in TK domain (6 %) encoded by exon 18, DD842 V mutation being the most frequent (65–75 %); and very rarely in exon 14 (0.1 %) [24, 25, 27, 33].
GIST wild type
Around 12–15 % of adult GIST and 90 % of pediatric GIST lack KIT and PDGFRA mutations [19]. Molecular pathogenesis and tumor biology of this subgroup represent one of the greatest areas of speculation and investigation in which the involvement of other TK receptors, such as IG1R, has already been demonstrated [34]. Besides, other intracellular signaling pathways as the one controlled by BRAF, with mutations described in 7 % of wild-type GIST [35] and mutations in the succinate dehydrogenase enzymatic complex subunit genes (SDHC), are mostly associated with germline mutations [36].
Kit-negative GIST
Approximately 5 % of GIST are c-kit negative, leading to diagnostic difficulties. Between 30 and 50 % of these tumors present mutations in KIT or PDGFRA [ 27, 37–39], which may have therapeutic implications. The notion that a GIST can be negative for c-kit as well as wild type for KIT and PDGFRA mutations is not entirely clear considering that current diagnosis is performed by exclusion [27]. Furthermore, the last European consensus proposed, using a mutational analysis of KIT and PDGFRA, to confirm GIST diagnosis, especially in CD117/DOG1 negative cases [40].
Syndromes associated with GIST
At present, there are many syndromes associated with GIST, among them:
-
1.
Carney Triad: characterized by gastric GIST, paraganglioma, pulmonary chondroma, which may develop in any age group, making it difficult to discard this condition in pediatric wild-type GIST [41].
-
2.
Neurofibromatosis Type 1: usually marked by a wild-type GIST predominantly located in the small intestine and quite possibly a multicenter study [42].
-
3.
Carney–Stratakis syndrome: characterized by germline mutations in some subunits of the succinate dehydrogenase enzyme (SDHB, SDHC y SDHD) producing a dyad of GIST and paraganglioma [43, 44].
Final recommendations
The last multidisciplinary ESMO consensus [40] recommends including a molecular systematic analysis in the diagnosis of all GIST (specially advanced GIST), given the type of relevant predictive and prognostic information provided and required in cases of GIST without CD117 and DOG1 expression. In these cases, it is recommended to refer patients to a reference center with their own laboratory, integrated in quality assurance programs and with proven experience.
Predictive factors in locally advanced or metastatic disease
Genotype correlation in primary disease with therapeutic results with imatinib for first line
Patients with an exon 11 KIT mutation have a better chance of responding, a longer time to progression (TTP) overall survival (OS) versus those with exon 9 mutations or wild type [45–47]. Moreover, the meta-analysis carried out in 2 phase II trials (EU-AUS and US-CDN) comparing 400 versus 800 mg of daily imatinib in patients with metastatic or non-resectable GIST, showed that patients with exon 11 mutation had a better progression-free survival (PFS) and overall survival with respect to exon 9 mutation or wild type [41].
Genotype correlation in primary disease with therapeutic results imatinib dosage
In the above-mentioned meta-analysis, it was found that mutations in exon 9 were the only predictive factor for imatinib response with greater benefit, statistically significant for PFS patients who received high doses of imatinib. A 31 % lower risk of death was observed in favor of 800 mg, although it did not reach statistical difference [48]. These findings confirm data previously disclosed by the European study. Unlike the American study, which only had 32 patients with exon 9 mutations, the European one showed 58 patients with same mutations [48, 49].
Primary genotype correlation with therapeutic results with sunitinib as second-line therapy
Sunitinib inhibits multiple receptor tyrosine kinases (VEGFR, PDGFR, KIT, FLT3) and has a higher receptor binding affinity than imatinib. Data obtained from Phase I/II clinical trials of 78 patients (out of 97) treated with sunitinib as second line and with preimatinib genotype information showed that patients with exon 9 mutations and wild-type tumor were associated with a more favorable outcome (partial response and disease stabilization over 6 months) compared with those with exon 11 mutations. Furthermore, the median PFS was significantly higher in patients with primary exon 9 mutation (19.4 months; p = 0.0005) or wild type (19 months; p = 0.0356) regarding those with exon 11 mutations (5.1 months). A significantly greater OS was obtained, comparing those with exon 9 mutations, (26.9 months; p = 0.012 and 30.5 months; p = 0.0132) for wild type, as opposed to those with exon 11 mutations (12.3 months) [49].
Secondary genotype correlation with therapeutic results for sunitinib
It is acknowledged that secondary resistance observed in patients with metastatic GIST is mostly developed due to the appearance of secondary mutations. Interestingly, no secondary mutations were found in initially wild type and resistant patients [50].
In vivo data obtained from biopsies carried out at the time of progression to imatinib provide important information on secondary mutations identified in 64 % of the available cases. Significant clinical benefit as well as RFS was better in cases with secondary mutations localized in exon 13 or 14 with regard to those in exon 17 or 18 [12].
Mechanisms of resistance to imatinib
Resistance to imatinib is a major therapeutic problem as, among patients who fail to respond to initial imatinib (primary resistance, 5–15 %) or stop responding (secondary resistance), barely 5 % respond to conventional treatments. Primary resistance can be defined as that occurring in the first months of imatinib therapy. Progression is typically multifocal, and mutations are frequently found in exons 9 and WT. The mechanisms for secondary resistance to imatinib are heterogeneous and can be grouped into various groups [51, 52]:
-
Acquisition of secondary KIT mutations
-
KIT gene amplification
-
Activation of alternative signaling pathways
-
Functional resistance due to KIT or PDGFRA activation in absence of secondary mutations.
The most common mechanism is the appearance of a new mutation. Secondary mutations in exons 13, 14, 17 or 18 account for 62 % of GIST with primary in KIT exon 11 mutations but only in 16 % have a primary mutation of exon 9. Moreover, no secondary mutations appear in GIST which do not have a primary mutation in KIT or PDGFRA [49]. There is evidence of clonal and/or polyclonal evolution of secondary mutations in a small proportion of patients (18.8 %). In this way over time, the same patient may develop secondary mutations in different tumor resistant implants. In view of this finding, the therapeutic approach of these patients will have to be taken into account [50, 53].
Final recommendations
The clinical application of secondary mutation findings is not so clear-cut at the present time and should be subject to further research studies.
Lastly, given the interest in translational studies regarding this neoplasm, it is advised to take fresh tissue for the application of new technologies of molecular pathology, which will ultimately have a positive impact on the patient.
Treatment
It is highly advised that diagnostic and therapeutic processes could be referred to expert teams for GIST care [54].
Localized disease
Surgery for localized disease
Complete surgical resection is the standard treatment for localized GIST. Radiological criteria for unresectability include infiltration of the celiac trunk, the superior mesenteric artery or mesenteric artery-to-portal vein. Lymphadenectomy is unnecessary given the low frequency of lymph node metástasis or metastasis. Some exceptions could be SDH-deficient GIST especially in pediatric population.
The aim was to achieve a R0-type surgery (optimal surgery), complete removal leaving an intact capsule. It is therefore necessary, in some cases, to remove neighboring organs and perform a surgical “block excision.” Segmental resection of intestine and stomach is accepted, and thus, aggressive and a more extensive surgery to remove unaffected tissue is unnecessary [55].
Regarding R1 resection (marginal excision containing tumor cells), reexcision could be offered and shared with the patient, if this does not imply major functional squeals. If the context of R1 surgery is a very low- to low-risk tumor, the physician should communicate the wait-and-see approach to the patient as opposed to aggressive surgery with permanent damage since there is no clear evidence that R1 margins entail a worse prognostic in such cases.
Peritoneal and hepatic surfaces should be carefully examined during a laparotomy to rule out tumor spread. Tumor resection must be carefully performed to avoid tumor rupture. In this regard, a laparoscopic approach is strongly discouraged in patients with voluminous tumors.
Prognostic factors after surgery in localized GIST
Relapse-risk assessment for primary GIST is paramount not only providing prognostic information when trying to determine risk factors but also estimating the potential benefit of adjuvant imatinib. In 2002, an index was proposed (NIH Consensus NIH or Fletcher) [15] based on studies of prognostic factors studies for patients with localized GIST, to estimate the risk of recurrence (Table 2; Fig. 1), based on the number of mitosis per 50 high-power fields (HPF), the size of the primary tumor and the two variables with the greatest prognostic significance. Principally, it seems that any GIST has malignant potential and the index makes it possible to classify GIST patients according to risk factors and complete resection.
Subsequently, Miettinen et al., analyzed data of 1.765 patients with gastric GIST and observed that patients only developed metastasis in 2–3 % tumors with <10 cm and <5 mitosis/50 HPF, compared with 68 % of those who presented >10 cm and >5 mitosis/50 HPF [10]. A second series including 906 patients with <10 cm and <5 mitosis/50 HPF tumor located in the jejunum and ileum, presented recurrence in 24 % compared to 90 % which presented >10 cm and >5 mitosis/50 HPF tumor.
Based on this data, these same authors put forward a new risk index (AFIP/Miettinen) that includes anatomic site [23]. This classification better reflects the high-risk population than the Fletcher index (Table 3; Fig. 2), especially between the intermediate and low-risk groups. The risk of gastric cancer relapse varies from 2 % in tumors with <5 mitosis per 50 HPF to 90 % in gastrointestinal tract GIST with tumors more than <10 cm and <5 mitosis/50 HPF. The casuistry of GEIS group has shown that the Miettinen’s classification exhibited statistical significance for discriminating low, intermediate and high-risk groups. This was not the case when the Fletcher classification was used [29].
The main differences between both classification systems lies in patients with gastric GIST, larger than 10 cm but with <5 mitosis per HPF. Using Fletcher’s classification, the latter would be in the high-risk group with a recurrence-free survival (RFS) of 50 % at 5 years. Nevertheless, they would fall within the intermediate-risk category with a RFS of 80 % according to the Miettinen group classification.
On the other end of the spectrum we find GIST tumors with extragastric location of <5 cm and more than 5 mitosis per HPF. According to Fletcher’s classification, they would fall within the intermediate group with a RFS probability of 85 % versus being in the high-risk group with 45 % RFS in the Miettinen group classification. It is important to note that Miettinen considered a total area 5 mm2 in 50 fields HPF characterized by the use of different optical components, while in practice 50 HPF typically corresponds to a total area of 10 mm2. Therefore, if we use Miettinen’s risk classification, we should also make the correction of dividing the number of mitosis by half including the current optical elements by 50 HPF.
Other succeeding risk classifications such as the American Joint Committee on Cancer (TNM) [56] or the nomogram [57] for the individual risk assessment show some differences such as the anecdotal evidence of ganglionic extension or the selection bias that encumber studies in some large centers and magnify the likelihood of relapse.
Joensuu H. recently introduced a capsule rupture classification known as modified NIH that simplifies the site classification (gastric/non-gastric) but at the same time renders heat maps to be more complex as categorization of continuous variables is not used [58].
The NCCN [59] and ESMO [40] guidelines tend to favor Miettinen’s classification when capsular rupture is considered comparable to peritoneal dissemination.
A further problem posed, at least theoretically, is regarding adjuvant imatinib clinical trials designed using Fletcher’s risk classification. If we were to adopt a more liberalized stance on drugs, we would recommend adjuvant imatinib treatment for patients with gastric GIST for tumors of 10 cm or larger with <5 mitosis/HPF (considered as high risk according to Fletcher), when the risk of recurrence is 65 %. Therefore, the most rational approach should bear in mind the most current prognostic information in which the high risk of recurrence category is more accurate.
Although GIST tumors are a model for the so-called molecular target therapies, molecular prognostic factors have not been incorporated in the risk of recurrence classifications. There is available evidence indicating that the type and location of the mutation has an effect on the risk of recurrence. Deletions affecting exon 11, codon 557/558 of the c-KIT gene, have a higher recurrence risk and it will occur within the first 3–4 years after surgery [29, 60]. The leading role of “critical mutation” has been confirmed in recent series [58, 61].
Final recommendations
-
We recommend the use of the risk group classification proposed by Miettinen as it is the best at identifying low, intermediate and high-risk populations. Spontaneous or intraoperative capsule rupture should be considered as a very poor prognostic factor.
-
Deletion type of mutations affecting codons 557 and 558 confers a risk for recurrence regardless of its previous classification, according to our experience. The risk is greatest within the first 30 months after surgery and then drops drastically. Nevertheless, results from other prospective studies are needed in order to assess the value of this variable.
Adjuvant treatment
Imatinib trials overview
Despite the fact that complete resection is feasible in most localized GIST cases, there is still a recurrence rate of up to 50 % according to some series. The role of imatinib as adjuvant treatment to prevent recurrence has therefore been assessed in several clinical trials. Evidence derived from the large Phase III randomized trials ACOSOG Z9001 [62] and SSGX-VIII/AIO [63], has shown a relapse-free survival (RFS) benefit with imatinib. Moreover, the SSGX VIII/AIO study showed an increase in overall survival (OS) with 3 years of imatinib administration over 1 year in high-risk patients (in accordance with NIH modifications). Preliminary results of the first interim analysis of EORTC 62024/GEIS-10 study [64] have recently been communicated in ASCO 2013. This phase III trial included intermediate and high-risk patients randomized at 2 years with imatinib over observation.
Although the initial end point was OS, it was changed to time to imatinib failure (TIF) in 2009 due to the small number of relapses in the control group. No significant statistical differences were found in either arms (OS and TFI) after a 4.7-year follow-up. Nevertheless, there was an objective tendency to an improved TFI in high-risk patients (in both NIH 2002 as well as modified NIH classifications). A benefit was also observed in RFS as previous reported studies in favor of adjuvant treatment with imatinib in high-risk patients.
In view of these results, both NCCN and ESMO guidelines as well as consensus of the scientific community, recommend 3 years of adjuvant treatment with imatinib in high-risk patients. Adjuvant treatment for low-risk patients is not indicated. However, currently there is not enough scientific evidence to support adjuvant treatment with imatinib in intermediate-risk patients. Based on these considerations, for uncertain cases, it is important to carry out an assessment of risk of recurrence and properly classify them by using modified classification tools (modified Miettinen classification of Joensuu H).
There are still many unclear areas concerning duration of adjuvant treatment and whether more than 3 years of treatment would increase benefit in patients at higher risk. Studies like PERSIST-5 may shed some light on this issue. Moreover, another aspect that needs to be clarified is whether relapse is actually avoided or just delayed, given the relapses observed in SSGX-VIII/AIO following adjuvant treatment interruption at 6–12 months in both arms [63].
Special cases
-
Capsule break: These are generally accepted as disseminated patients given that 100 % will relapse, at least on a peritoneal level. Therefore, imatinib administration is recommended as advanced disease setting.
-
Specific genotypes: Adjuvant imatinib is not recommended in patients with D842 V PDGFRα mutation given its known resistance to it. There is no consensus regarding the benefit of a daily dose of 400 mg of imatinib for carriers of an exon 9 mutation in the KIT gene. The efficacy of a daily dose of 800 mg of imatinib was extrapolated from the evidence of disseminated disease. Nonetheless, this scenario has not been proven in clinical trials and therefore has not been approved for adjuvant treatment. Survival in patients with wild type does not seem to increase with the use of adjuvant imatinib, and thus, there is still controversy over imatinib administration and each case must be considered individually.
-
Patients with R1 surgery: There is no evidence confirming the benefit of adjuvant imatinib in low-risk patients with affected microscopic margins. Surgical reexcision could be considered for these cases (see surgical section).
Final recommendations
-
High-risk patients: 3 years of adjuvant treatment with imatinib is recommended.
-
Low-risk patients: adjuvant treatment is not indicated.
-
Intermediate-risk patients: currently, there is not enough scientific evidence to support adjuvant treatment with imatinib. For uncertain cases, it is important to carry out an assessment of risk of recurrence and properly classify them by using modified classification tools (modified Miettinen classification of Joensuu H).
Advanced disease
Treatment of unresectable or metastatic disease
Dose and efficacy of imatinib treatment
Gastrointestinal stromal tumors have been a paradigmatic example of chemo-resistant tumors with <5 % of responses and 14 months as the median of survival reported in the literature. Imatinib mesylate (STI571, Gleevec™, Novartis Pharmaceuticals, Basel, Switzerland) is a selective tyrosine kinase inhibitor (TKI), whose targets include ABL, BCR-ABL, KIT and PDGFR and constitutes as a very effective agent for the treatment of clinically advanced, metastatic or surgically unresectable GIST [65, 66].
The standard dose of imatinib of 400 mg/m2 per day was established from two different randomized phase III trials in metastatic GIST with positive immunostaining for kit (EORTC-ISG-AGITG y NASG-S0033). In both trials, daily doses of 400 versus 800 mg were compared without any survival difference and with a more favorable toxicity profile for lower doses. The clinical benefit rates (CR, PR and SD) for 400 and 800 mg were 90 and 88 %, respectively, in NASG-S0033 study. These rates were 91 and 87 %, respectively, in EORTC-ISG-AGITG study. Furthermore, there was statistically significant difference, in terms of progression-free survival (PFS), favoring 800 mg dose in an European trial: progression-free rate at 2 years 52 versus 44 % (HR 0.78) [67, 68]. In a meta-analysis, analyzing 1,640 patients enrolled in the mentioned trials, a slight but still significantly advantage was found in terms of PFS for the high-dose arm [48]. Nevertheless, no survival advantage was detected and thus the standard dose, as for general recommendation, is 400 mg daily.
Predictive value of genotype for imatinib efficacy
Interestingly, one of the notable features of the clinical studies of imatinib for GIST treatment is the consistent observation that defined subsets of GIST according to their mutational status have different outcomes during treatment and therefore should be considered in devising treatment strategies.
Responses to imatinib depend on the functional domain affected [69]. Table 4 lists the correlation between tumor genotype and objective response (both complete and partial responses) in four trials (phase I–III). On the basis of 768 genotyped GIST, the objective response rates for KIT exon 11, exon 9 mutants and GIST WT are 72, 38 and 28 %, respectively [46, 70, 71]. Likewise, the probabilities of primary resistance to imatinib for KIT exon 11, KIT exon 9 and WT GIST are 5, 16 and 23 %, respectively (Table 4). An even more striking observation is that KIT and PDGFRA mutational status correlates with time to progression (TTP) and overall survival (OS), with superior survival seen for patients with GIST carrying an exon 11 KIT mutation. For example, in the American phase III trial, the median TTP for patients with GIST harboring KIT exon 11, KIT exon 9 and WT was 25, 17 and 12, 8 months, respectively. A similar OS benefit was seen for patients with KIT exon 11 mutations (60 months) compared with those observed for KIT exon 9 (38 months) or WT (49 months) genotypes. Comparable results regarding TTP, OS and KIT mutational status were also observed in the European/Australasian phase III trial [46].
On the other hand, the meta-analysis also confirmed the observations previously reported in the European/Australasian trial, and therefore, it was concluded that KIT exon 9 mutations constituted a dose-dependent predictive factor for imatinib treatment identifying patients with a better response to high doses of imatinib (400 mg twice daily). Consequently, the estimated risk of progression for patients with KIT exon 9 mutations was drastically reduced (42 %; p = 0.0017) in the 800-mg/day arm compared with the 400-mg/day dose of imatinib. In the same direction, the risk of death was also reduced in a 31 % in this subgroup of patients.
Only small numbers of patients with GIST harboring PDGFRA mutations were included in the original phase I–III trials. On the basis of in vitro data, the most common PDGFRA mutation in GIST, D842 V, is fully resistant to the effects of imatinib [33]. Among the patients whose GIST harbored a PDGFRA D842 V mutation in the American phase III trial, there were no objective responses and stable disease was observed for a few months in some of the patients. From in vitro experiments, dasatinib showed activity in GIST cell lines with this specific mutation [72], somewhat recently confirmed in the clinical setting [73].
Thus, taking together the previous information, there is a consensus in the following recommendations:
-
Genotype is mandatory for treating advanced/metastatic GIST patients. Evidence II, A.
-
Imatinib 400 mg/day is the recommended dose in first line in advanced/metastatic GIST. Evidence I, A.
-
In exon 9 mutants, imatinib 800 mg/day is the recommended dose. Evidence III, A.
-
In PDGFRA/KIT WT GIST is not clear enough that imatinib should be the standard.
-
In imatinib-resistant D842 V mutant, alternative treatments other than imatinib could be advised (i.e., dasatinib). Evidence IV, B.
Practical issues on imatinib as first line in GIST
-
1.
How long should the therapy last? The BFR14 trial, which randomized patients with nonprogressive GIST to continuation versus interruption of imatinib after 1, 3, or 5 years of treatment, showed that treatment interruption was associated with a high risk of progression even in patients with a complete response [13]. Interestingly, although imatinib rechallenge could control the disease in most patients, the quality of the tumor response rarely reached that before treatment interruption. Consequently, in patients with metastatic or unresectable GIST, imatinib should be continued until disease progression even when metastatic lesions have been previously surgically excised or until unacceptable toxicity. Evidence II, B.
-
2.
Compliance. Although imatinib is usually a well-tolerated drug with as few as 2 % of grade III–IV adverse events, the long duration of therapy and persistent grade I–II side effects could impact in treatment compliance and consequently in disease outcome. Therefore, a good education of patients regarding the importance of compliance and potential interactions with other drugs or foods as well a proper and prompt management of side effects is crucial.
-
3.
Imatinib plasma levels. Although it remains to be demonstrated in a prospective setting, retrospective data suggest that low plasma levels at steady state are associated with a worse outcome. So, the median time to progression was 11.3 months for patients with imatinib plasma levels <1, 11 ng/mL compared to more than 30 months for patients with plasma levels above that threshold [14]. Plasma levels could be especially useful in case of suspected bad compliance as the cause of tumor progression, in patients at risk of potentially important interactions with other concomitant drugs or unexpected toxicities. Evidence IV, B.
-
4.
Rechallenge of imatinib after adjuvant treatment. For patients recurring during adjuvant treatment, second-line treatments including imatinib 800 mg/day should be discussed. For those patients relapsing with metastatic or unresectable disease after imatinib interruption, although no direct prospective evidence is available, based on the data from the previously mentioned BRF14 trial, the general recommendation is that imatinib should be reintroduced at the same dose as recommended for first line. Evidence II, B.
Treatment for patients with disease progression following imatinib failure
The first step to undertake when dealing with advanced GIST patients, who have progressed despite imatinib treatment, is to ensure treatment adherence and check for drug interactions that might decrease efficacy. Consideration may also be given to determine plasma imatinib concentrations, to better analyze these issues [78]. If there is proper treatment compliance, systemic treatment will have to be modified.
Imatinib dose escalation. The first recommended therapeutic maneuver consists of increasing the dose of imatinib to 800 mg/daily. The decision is based on results of crossover to 800 mg after disease progression on 400 mg in EORTC phase III trial studies [79] and American Intergroup (study S0033) [68]. In both cases, the observed progression-free rates in patients receiving a higher dosage were 30–35 %. The median time to progression was 3–4 months. However, in one of the studies, 18 % of patients remained progression free during 1 year. The incidence of anemia and asthenia increases significantly with this dosage; therefore, a strict follow-up is required. Results from a retrospective EORTC trial indicated that 800 mg dose is more effective than 400 mg in patients who have KIT exon 9 mutations [46]. These data were published recently in a meta-analysis including 722 patients [48].
Sunitinib is a multitargeted or selective TKI active inhibitor that is active against alpha-type and beta-type PDGFR and VEGFR receptors. Results of a randomized phase III trial versus placebo revealed a prolongation of the time to progression from 1.5 to 6.3 months in patients with GIST who progressed despite imatinib treatment [80]. Accordingly, it has been approved by the EMA and FDA as treatment for GIST resistant to imatinib therapy and for those who do not tolerate it. The recommended dose is 50 mg orally once a day during 4 weeks followed by a 2-week rest period, although an uninterrupted daily dose of 37, 5 mg is a valid alternative [81]. The most common side effects were asthenia, skin toxicity, diarrhea, hypertension and hypothyroidism. A prospective study showed an increased drug efficacy in patients with wild-type KIT GIST or mutations in exon 9 and 11 [49]. Likewise, patients who benefited most from sunitinib treatment were those with secondary KIT mutations in exon 13 and 14 compared to those with exon 17 and 18 mutations.
Regorafenib is a second generation TK inhibitor targeting KIT, RET, BRAF, VEGFR, PDGFR and FGFR. A median PFS of 4.8 months with imatinib, sunitinib and regorafenib versus 0.9 months with placebo was observed in a randomized trial for patients with refractory GIST. The most frequent side effects were high-blood pressure, hand-foot syndrome and diarrhea [82]. The only available preliminary data showed a correlation between genotype and regorafenib sensitivity, suggesting significant activity against KIT exon 11 mutations and those found in the KIT-activating mutations loops as well as some forms of KIT wild type. Regorafenib has the FDA approval and is currently undergoing assessment by the EMA as treatment for advanced GIST after failure of imatinib and sunitinib.
The available treatment options following imatinib, sunitinib and regorafenib administration (the latter following approval of regulatory authorities) are still in the experimental phase. The first recommendation is to offer these patients the opportunity to participate in clinical trials conducted under an investigational new drug. If this is not possible, an individual treatment can be scheduled for selected patients with other drugs such as:
Nilotinib is a second generation TK inhibitor active in chronic myeloid leukemia and with the inhibitory effect of KIT and PDGF. Preliminary results of a phase III trial comparing nilotinib with imatinib, sunitinib or supportive care in resistant GIST patients whose disease had progressed to imatinib and sunitinib did not show differences in PFS and OS treatment groups. On the other hand, significant differences in overall survival were noted when the analyses were limited to those who received nilotinib as strictly third-line treatment (excluding those who had received additional therapy) [83].
Sorafenib is a VEGFR, KIT, PDGR and BRAF inhibitor. Preliminary results of a phase II trial showed activity in imatinib-resistant and sunitinib-resistant patients with acceptable tolerance. This could be an alternative until regorafenib becomes available [84].
Imatinib and doxorubicin combination: Promising activity was been observed in a GEIS group study with doxorubicin dose of 20 mg/m2/once a week, which could be particularly suitable for patients with wild-type GIST [85].
Response evaluation
An abdominal and pelvic CT with contrast and image acquisitions of the arterial and portal phases allows identification of hypervascular hepatic lesions that would otherwise go unnoticed and become evident when they become hypodense with treatment. Choi criteria [74] combine changes in both size (RECIST) and density measures (Hounsfield Units: HU). Responses can mimic progression due to the increasing size of some lesions that can only be interpreted if HU are considered [75]. On the contrary, a CT scan without endovenous contrast detects hemorrhage or intratumoral calcification.
Other techniques such as MRI are strictly limited to hepatic studies, complex locations such as the rectum [76] and allergic reactions to iodine contrast, since evaluation of HU is not feasible. PET is reserved for inconclusive cases by other techniques such as CT or MRI or the early assessment of response to imatinib [77]. However, PET is useful for early detection of responses, mandatory in some neo-adjuvant indications.
Both RECIST 1.1 and Choi [74] criteria must be taken into account to avoid confounding it with pseudoprogression due to myxoid degeneration or intratumoral hemorrhage (Table 5).
Follow-up of patient diagnosed with gist
An abdomen and pelvic CT with contrast and image acquisitions of the arterial and portal phases is the method of choice for initial diagnosis and follow-up for GIST. Due to low metastatic frequency of pulmonary metastases (2 %) [86], thoracic imaging study is only indicated based on clinical suspicion. There are no studies analyzing the efficacy of different follow-up strategies. Follow-up will be stratified based on risk, size, number of mitosis and location according to the Miettinen classification [9].
Other techniques such as MRI are strictly limited to hepatic studies, complex locations such as the rectum and allergic reactions to iodine contrast given that evaluation of Hounsfield units is not feasible. PET is reserved for inconclusive cases by other techniques such as CT or MRI or the early assessment of response to imatinib [77].
Localized resectable disease
Follow-up after resection according to risk group:
Very low risk: Routine follow-up is not indicated due to the low-risk of relapse- even if it is not null.
Low risk: Follow-up can be carried out every 6 months at diagnosis up to the fifth year.
Intermediate high risk: Patients receiving adjuvant imatinib treatment require regular monitoring with clinical analysis every 3 months to control drug tolerance. A follow-up CT scan should be done every 3–6 months during adjuvant treatment. The highest risk of recurrence takes place in the first 2 years. After this period, a CT scan should be performed every 3–6 months up to the fifth year and annually up to 10 years.
Localized unresectable or metastatic disease
Follow-up should be conducted every 3 months since the beginning and can be prolonged up to every 6 months if response is obtained, especially if response remains beyond a 5-year period. Both RECIST 1.1 and Choi [74] criteria must be taken into account to avoid confounding it with pseudoprogression due to myxoid degeneration or intratumoral hemorrhage as previously described.
Special cases
Neoadjuvant and induction therapy
Systemic induction therapy has the aim of facilitating surgery through tumor shrinkage, whereas systemic neoadjuvant therapy targets survival advantage [87].
In locally advanced and unresectable GIST, there are few cases that would eventually become resectable after induction treatment with imatinib [88]. However, for those locally and resectable GIST for which a mutilating surgery is planned, a cytoreductive treatment with imatinib should be attempted. Thus, in gastric GIST to avoid subtotal gastrectomy, duodenal GIST near the ampulla of Vater or rectal GIST to avoid abdominoperineal amputation.
Early assessment of response is needed to minimize risks since a delay could further hamper surgery after an unsuccessful imatinib treatment. Mutational analysis should be mandatory due to the robust genotype predictive biomarker (IIb). Hence, exon 9 mutants would require 800 mg/day of imatinib; D842 V mutants no induction treatment would be active and for those KIT/PDGFRA wild type, is doubtful that imatinib could be active enough. A CT scan can be of some usefulness for assessing early response, but the use of PET scan after induction therapy seems more advantageous given its ability to verify the efficacy of the treatment within a very short time [89].
The recommended duration of preoperatory treatment cannot be based on objective criteria. However, it is estimated that surgery could be performed within 6–12 months after starting imatinib, since maximal response and minimal risk of secondary resistance is expected in this time interval.
Final recommendations
-
1.
There is a lack of published evidence regarding neoadjuvant treatment in operable GIST and therefore should not be used outside clinical trials.
-
2.
Induction treatment could be recommended in individual basis with the aim of offering a less mutilating/function sparing surgery.
Small GIST<2 cm
Many small GIST, <2 cm, are incidental findings during surgeries carried out for a number of other reasons. A small GIST found accidentally in a surgical specimen does not require any additional therapeutic procedure.
In those uncommon cases of small GIST diagnosed before surgery, the excision is not clear enough and a shared decision-making process with the patient should be offered.
Anyway, the mitotic index of those tumors should be taken into account, although the incidence of small size and high mitotic index is very low according the literature.
Focal progression
There is a well-documented type of secondary resistance to imatinib called nodule within mass. This is a focal disease progression, while most of the tumor burden is still under control. For these cases, maintenance of systemic treatment (generally imatinib) as well as applying local measures, can keep the patient free of progression for over a year in one-third of cases (xii). It is the treatment of choice over second-line systemic treatments.
The most common local treatments are surgery, radioablation and arterial embolization. In the absence of controlled trials, the choice of treatment should be based disease characteristics as well as on an experienced medical team.
Final recommendations
-
Systemic therapy should not be interrupted or replaced when progression is limited to a single or only a few focal points amenable to local treatment.
-
Choice treatment for focal progression is maintenance of systemic therapy together with local control techniques appropriate for each case.
References
Miettinen M, Lasota J (2001) Gastrointestinal stromal tumors: definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch Int J Pathol. 438(1):1–12. PubMed PMID: 11213830
Lau S, Tam KF, Kam CK, Lui CY, Siu CW, Lam HS, et al (2004) Imaging of gastrointestinal stromal tumour (GIST). Clin Radiol.59(6):487–498. PubMed PMID: 15145718
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG et al (2010) NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Comp Cancer 8 (Suppl 2):S1-41; quiz S2-4. PubMed PMID: 20457867
Lee CM, Chen HC, Leung TK, Chen YY (2004) Gastrointestinal stromal tumor: computed tomographic features. World J Gastroenterol 15;10(16):2417–2418. PubMed PMID: 15285033
Casali PG, Blay JY, Experts ECECPo (2010) Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v98–v102
Bensimhon D, Soyer P, Boudiaf M, Fargeaudou Y, Nemeth J, Pocard M et al (2009) Imaging of gastrointestinal stromal tumors. J Radiol 90(4):469–480. PubMed PMID: 19503028
Tio TL, Tytgat GN, den Hartog Jager FC (1990) Endoscopic ultrasonography for the evaluation of smooth muscle tumors in the upper gastrointestinal tract: an experience with 42 cases. Gastrointest Endosc 36(4):342–350
Lamba G, Gupta R, Lee B, Ambrale S, Liu D (2012) Current management and prognostic features for gastrointestinal stromal tumor (GIST). Exp Hematol Oncol 1(1):14. PubMed PMID: 23210689. Pubmed Central PMCID: 3514103
Miettinen M, Majidi M, Lasota J (2002) Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer 38(Suppl 5):S39–S51
Miettinen M, Lasota J, Sobin LH (2005) Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol 29(10):1373–1381. PubMed PMID: 16160481. Epub 2005/09/15. eng
Rubin BP (2006) Gastrointestinal stromal tumours: an update. Histopathology. 48(1):83–96. PubMed PMID: 16359540
Gasparotto D, Rossi S, Bearzi I, Doglioni C, Marzotto A, Hornick JL et al (2008) Multiple primary sporadic gastrointestinal stromal tumors in the adult: an underestimated entity. Clin Cancer Res 15;14(18):5715–5721. PubMed PMID: 18779314
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130(10):1466–1478
Lin SC, Huang MJ, Zeng CY, Wang TI, Liu ZL, Shiay RK (2003) Clinical manifestations and prognostic factors in patients with gastrointestinal stromal tumors. World J Gastroenterol 9(12):2809–2812
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ et al (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33(5):459–465. PubMed PMID: 12094370. Epub 2002/07/03. eng
Rubin BP, Blanke CD, Demetri GD, Dematteo RP, Fletcher CD, Goldblum JR et al (2010) Protocol for the examination of specimens from patients with gastrointestinal stromal tumor. Arch Pathol Lab Med 134(2):165–170. PubMed PMID: 20121601
Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B et al (2005) Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era–a population-based study in western Sweden. Cancer 15;103(4):821–829. PubMed PMID: 15648083
Liegl B, Hornick JL, Corless CL, Fletcher CD (2009) Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol 33(3):437–446. PubMed PMID: 19011564
Debiec-Rychter M, Wasag B, Stul M, De Wever I, Van Oosterom A, Hagemeijer A et al (2004) Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J Pathol 202(4):430–438
Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC et al (2004) KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 28(7):889–894
Lee HE, Kim MA, Lee HS, Lee BL, Kim WH (2008) Characteristics of KIT-negative gastrointestinal stromal tumours and diagnostic utility of protein kinase C theta immunostaining. J Clin Pathol 61(6):722–729
Rossi G, Valli R, Bertolini F, Marchioni A, Cavazza A, Mucciarini C et al (2005) PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft-tissue tumours of the gastrointestinal tract. Histopathology 46(5):522–531
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23(2):70–83. PubMed PMID: 17193820. Epub 2006/12/30. eng
Reichardt P, Hogendoorn PC, Tamborini E, Loda M, Gronchi A, Poveda A et al (2009) Gastrointestinal stromal tumors I: pathology, pathobiology, primary therapy, and surgical issues. Semin Oncol 36(4):290–301
Lasota J, Miettinen M (2008) Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 53(3):245–266
Martin-Broto J, Rubio L, Alemany R, Lopez-Guerrero JA (2010) Clinical implications of KIT and PDGFRA genotyping in GIST. Clin Trans Oncol 12(10):670–676. PubMed PMID: 20947481
Corless CL, Heinrich MC (2008) Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 3:557–586
Rubin BP, Heinrich MC, Corless CL (2007) Gastrointestinal stromal tumour. Lancet 9;369(9574):1731–1741. PubMed PMID: 17512858
Martin J, Poveda A, Llombart-Bosch A, Ramos R, Lopez-Guerrero JA, Garcia del Muro J et al (2005) Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 1;23(25):6190–6198. PubMed PMID: 16135486. Epub 2005/09/02. eng
Corless CL, Fletcher JA, Heinrich MC (2004) Biology of gastrointestinal stromal tumors. J Clin Oncol 15;22(18):3813–3825. PubMed PMID: 15365079
Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, et al (2000) Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors: a study of 200 cases. Am J Pathol 157(4):1091–1095. PubMed PMID: 11021812. Pubmed Central PMCID: 1850182
Antonescu CR, Sommer G, Sarran L, Tschernyavsky SJ, Riedel E, Woodruff JM, et al (2003) Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res 15;9(9):3329–3337. PubMed PMID: 12960119
Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P et al (2005) PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 10;23(23):5357–5364. PubMed PMID: 15928335
Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, et al (2008) Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci USA 17;105(24):8387–8392. PubMed PMID: 18550829. Pubmed Central PMCID: 2448846
Agaimy A, Terracciano LM, Dirnhofer S, Tornillo L, Foerster A, Hartmann A et al (2009) V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Pathol 62(7):613–616
Pantaleo MA, Astolfi A, Urbini M, Nannini M, Paterini P, Indio V et al (2014) Analysis of all subunits, SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complex in KIT/PDGFRA wild-type GIST. Eur J Hum Genet 22(1):32–39. PubMed PMID: 23612575. Pubmed Central PMCID: 3865408
Pauls K, Merkelbach-Bruse S, Thal D, Buttner R, Wardelmann E (2005) PDGFRalpha- and c-kit-mutated gastrointestinal stromal tumours (GISTs) are characterized by distinctive histological and immunohistochemical features. Histopathology 46(2):166–175
Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y et al (2003) Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 125(3):660–667
Wasag B, Debiec-Rychter M, Pauwels P, Stul M, Vranckx H, Oosterom AV et al (2004) Differential expression of KIT/PDGFRA mutant isoforms in epithelioid and mixed variants of gastrointestinal stromal tumors depends predominantly on the tumor site. Mod Pathol 17(8):889–894
ESMO/European Sarcoma Network Working Group (2012) Gastrointestinal stromal tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23 Suppl 7:vii49–vii55. PubMed PMID: 22997454. Epub 2012/11/20
Zhang L, Smyrk TC, Young WF Jr., Stratakis CA, Carney JA (2010) Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol 34(1):53–64. PubMed PMID: 19935059. Pubmed Central PMCID: 3652406
Miettinen M, Fetsch JF, Sobin LH, Lasota J (2006) Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 30(1):90–96. PubMed PMID: 16330947
Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M et al (2008) Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet 16(1):79–88
Gaal J, Stratakis CA, Carney JA, Ball ER, Korpershoek E, Lodish MB et al (2011) SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol 24(1):147–151. PubMed PMID: 20890271. Pubmed Central PMCID: 3415983
Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD et al (2008) Correlation of kinase genotype and clinical outcome in the North American Intergroup phase III trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol 20;26(33):5360–5367. PubMed PMID: 18955451. Pubmed Central PMCID: 2651078
Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT et al (2006) KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42(8):1093–1103. PubMed PMID: 16624552
Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA et al (2008) Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 1;26(4):620–625. PubMed PMID: 18235121
Gastrointestinal stromal tumor meta-analysis G (2010) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 1;28(7):1247–1253. PubMed PMID: 20124181. Pubmed Central PMCID: 2834472
Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D et al (2008) Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 20;26(33):5352–5359. PubMed PMID: 18955458. Pubmed Central PMCID: 2651076
Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ et al (2006) Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 10;24(29):4764–4774. PubMed PMID: 16954519
Tamborini E, Bonadiman L, Greco A, Albertini V, Negri T, Gronchi A et al (2004) A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology 127(1):294–299
Chen LL, Trent JC, Wu EF, Fuller GN, Ramdas L, Zhang W et al (2004) A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res 1;64(17):5913–5919. PubMed PMID: 15342366
Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B et al (2005) Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Cancer Res 1;11(11):4182–4190. PubMed PMID: 15930355
Poveda A, Rivera F, Martin J, Spanish Group for Sarcoma R, Seom (2012) SEOM guidelines for gastrointestinal stromal sarcomas (GIST). Clin Transl Oncol 14(7):536–540. PubMed PMID: 22721799
Everett M, Gutman H (2008) Surgical management of gastrointestinal stromal tumors: analysis of outcome with respect to surgical margins and technique. J Surg Oncol 15;98(8):588–593. PubMed PMID: 19072850
Edge SB (2010) American joint committee on cancer. American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual. 7th edn. Springer, New York: xix, pp 718
Gold JS, Gonen M, Gutierrez A, Broto JM, Garcia-del-Muro X, Smyrk TC et al (2009) Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 10(11):1045–1052. PubMed PMID: 19793678. Pubmed Central PMCID: 3175638. Epub 2009/10/02. eng
Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P et al (2012) Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 13(3):265–274. PubMed PMID: 22153892. Epub 2011/12/14. eng
Von Mehren M, Benjamin RS, Bui MM, Casper ES, Conrad EU, 3rd, DeLaney TF et al (2012) Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Comp Cancer 10(8):951–960. PubMed PMID: 22878820. Epub 2012/08/11. eng
Wardelmann E, Losen I, Hans V, Neidt I, Speidel N, Bierhoff E et al (2003) Deletion of Trp-557 and Lys-558 in the juxtamembrane domain of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. International journal of cancer. Int J Cancer 10;106(6):887–895. PubMed PMID: 12918066. Epub 2003/08/15. eng
Corless CL (2010) Relation of tumor pathologic and molecular features to outcome after surgical resection of localized primary gastrointestinal stromal tumor (GIST): results of the intergroup phase III trial ACOSOG Z9001. J Clin Oncol 28:15 s(suppl; abstr 10006)
Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD et al (2009) Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 28;373(9669):1097–1104. PubMed PMID: 19303137. Pubmed Central PMCID: 2915459
Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schutte J et al (2012) One versus three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 28;307(12):1265–1272. PubMed PMID: 22453568
Casali PG, Le Cesne A, Poveda Velasco A, Kotasek D, Rutkowski P, Hohenberger P et al (2013) Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): the EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. ASCO Meeting Abstracts. 17, 2013;31(15_suppl):10500
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D et al (2001) Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 5;344(14):1052–1056. PubMed PMID: 11287975
Demetri GD (2002) Identification and treatment of chemoresistant inoperable or metastatic GIST: experience with the selective tyrosine kinase inhibitor imatinib mesylate (STI571). Eur J Cancer 38 Suppl 5:S52–S59. PubMed PMID: 12528773. Epub 2003/01/17. eng
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY et al (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 25-Oct 1;364(9440):1127–1134. PubMed PMID: 15451219
Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS et al (2008) Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 1;26(4):626–632. PubMed PMID: 18235122
Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H et al (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 1;21(23):4342–4349. PubMed PMID: 14645423
Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N et al (2003) PDGFRA activating mutations in gastrointestinal stromal tumors. Science 31;299(5607):708–710. PubMed PMID: 12522257. Epub 2003/01/11. eng
Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M et al (2004) Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 40(5):689–695. PubMed PMID: 15010069
Dewaele B, Wasag B, Cools J, Sciot R, Prenen H, Vandenberghe P et al (2008) Activity of dasatinib, a dual SRC/ABL kinase inhibitor, and IPI-504, a heat shock protein 90 inhibitor, against gastrointestinal stromal tumor-associated PDGFRAD842 V mutation. Clin Cancer Res 15;14(18):5749–5758. PubMed PMID: 18794084
Trent J (2011) A phase II study of dasatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST). J Clin Oncol;29(Suppl, abst 10006)
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR et al (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 1;25(13):1753–1759. PubMed PMID: 17470865. Epub 2007/05/02. eng
Sandrasegaran K, Rajesh A, Rushing DA, Rydberg J, Akisik FM, Henley JD (2005) Gastrointestinal stromal tumors: CT and MRI findings. Eur Radiol 15(7):1407–1414. PubMed PMID: 15761716
Jiang ZX, Zhang SJ, Peng WJ, Yu BH (2013) Rectal gastrointestinal stromal tumors: imaging features with clinical and pathological correlation. World J Gastroenterol 28;19(20):3108–3116. PubMed PMID: 23716991. Pubmed Central PMCID: 3662951
Trent JC, Ramdas L, Dupart J, Hunt K, Macapinlac H, Taylor E et al (2006) Early effects of imatinib mesylate on the expression of insulin-like growth factor binding protein-3 and positron emission tomography in patients with gastrointestinal stromal tumor. Cancer 15;107(8):1898–1908. PubMed PMID: 16986125
Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD et al (2009) Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol 1;27(19):3141–3147. PubMed PMID: 19451435
Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY et al (2005) Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer 41(12):1751–1757. PubMed PMID: 16098458
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J et al (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 14;368(9544):1329–1338. PubMed PMID: 17046465
George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE et al (2009) Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 45(11):1959–1968. PubMed PMID: 19282169
Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H et al (2013) Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 26;381(9863):295–302. PubMed PMID: 23177515. Pubmed Central PMCID: 3819942
Reichardt P, Blay JY, Gelderblom H, Schlemmer M, Demetri GD, Bui-Nguyen B et al (2012) Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol 23(7):1680–1687
Wiebe L, Kasza KE, Maki RG, D’Adamo DR, Chow WA, Wade III JL et al (2008) Activity of sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): a phase II trial of the University of Chicago Phase II Consortium. ASCO Meeting Abstracts. 18;26(15_suppl):10502
Maurel J, Martins AS, Poveda A, Lopez-Guerrero JA, Cubedo R, Casado A et al (2010) Imatinib plus low-dose doxorubicin in patients with advanced gastrointestinal stromal tumors refractory to high-dose imatinib: a phase I–II study by the Spanish Group for Research on Sarcomas. Cancer 1;116(15):3692–3701. PubMed PMID: 20564079
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231(1):51–58. PubMed PMID: 10636102. Pubmed Central PMCID: 1420965
Fiore M, Palassini E, Fumagalli E, Pilotti S, Tamborini E, Stacchiotti S et al (2009) Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol 35(7):739–745. PubMed PMID: 19110398
Bauer S, Hartmann JT, de Wit M, Lang H, Grabellus F, Antoch G et al (2005) Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. International journal of cancer. Int J Cancer 1;117(2):316–325. PubMed PMID: 15900603
McAuliffe JC, Hunt KK, Lazar AJ, Choi H, Qiao W, Thall P et al (2009) A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 16(4):910–919
Acknowledgments
The authors would like to thank Dr. Vicens Artigas, Dr. José Cervera, Dr. Juan R. Delgado, Dr. Manuel García de Polavieja, Dr. José Antonio López Martín, Dr. Antonio López Pousa, Dr. Luis Ortega, Dr. Rafael Ramos, Dra. Mª José Safont for helping with their opinions and MFAR S.L. for its administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of GEIS (Grupo Español de Investigación en Sarcomas/Spanish Group for Research on Sarcoma).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Poveda, A., del Muro, X.G., López-Guerrero, J.A. et al. GEIS 2013 guidelines for gastrointestinal sarcomas (GIST). Cancer Chemother Pharmacol 74, 883–898 (2014). https://doi.org/10.1007/s00280-014-2547-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2547-0