Abstract
Purpose
To review the prognostic factors and stratification systems used to determine the need for adjuvant therapy in the treatment of gastrointestinal stromal tumors (GIST), and to review recent clinical advances in investigation of the efficacy and safety of adjuvant imatinib mesylate treatment.
Methods
Recent data from clinical trials of various durations of adjuvant imatinib in GIST are reviewed, with emphasis on key results from the Phase III American College of Surgeons Oncology Group (ACOSOG) Z9001 trial and the Scandinavian Sarcoma Group XVIII/Arbeitsgemeinschaft Internistische Onkologie (SSGXVIII/AIO) trial.
Results
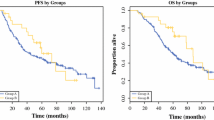
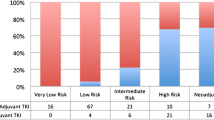
Complete surgical resection remains the standard of treatment for localized GISTs; however, disease recurrence occurs in up to 50 % of patients who undergo complete resection. The ACOSOG Z9001 trial established that 1 year of adjuvant imatinib reduces the risk of recurrence in patients with resected GIST. The SSGXVIII/AIO trial further demonstrated that 3-year adjuvant imatinib improves both recurrence-free survival and overall survival compared with 1-year therapy in patients at high risk of recurrence after surgery. Considering risk factors associated with tumor recurrence is essential for identifying the patients who are most likely to benefit from adjuvant imatinib.
Conclusions
Although the optimal duration of adjuvant therapy remains to be determined, results from these pivotal trials provide firm evidence that adjuvant imatinib improves recurrence-free survival and improved overall survival of patients in the SSGXVIII/AIO trial. Ongoing studies may shed further light on the benefits and harms of adjuvant therapy, as well as the most appropriate patient candidates for adjuvant imatinib treatment.
Similar content being viewed by others
References
Miettinen M, Sobin LH, Lasota J (2005) Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29:52–68
Miettinen M, Makhlouf H, Sobin LH, Lasota J (2006) Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 30:477–489
National Comprehensive Cancer Center (2012) NCCN clinical practice guidelines in oncology: soft tissue sarcoma V2.2012. http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. Accessed 22 Aug 2012
Casali PG, Blay JY (2010) Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v98–v102
DeMatteo RP, Lewis JJ, Leung D et al (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Gold JS, Gonen M, Gutierrez A et al (2009) Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 10:1045–1052
Maleddu A, Pantaleo MA, Nannini M, Biasco G (2011) The role of mutational analysis of KIT and PDGFRA in gastrointestinal stromal tumors in a clinical setting. J Transl Med 9:75
Hassan I, You YN, Shyyan R et al (2008) Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Ann Surg Oncol 15:52–59
Joensuu H, Vehtari A, Riihimaki J et al (2012) Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 13:265–274
DeMatteo RP, Heinrich MC, El Rifai WM, Demetri G (2002) Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 33:466–477
Rutkowski P, Wozniak A, Debiec-Rychter M et al (2011) Clinical utility of the new American Joint Committee on Cancer staging system for gastrointestinal stromal tumors: current overall survival after primary tumor resection. Cancer 117:4916–4924
Joensuu H, Roberts PJ, Sarlomo-Rikala M et al (2001) Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 344:1052–1056
Demetri GD, von Mehren M, Blanke CD et al (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472–480
Eisenberg BL, Trent JC (2011) Adjuvant and neoadjuvant imatinib therapy: current role in the management of gastrointestinal stromal tumors. Int J Cancer 129:2533–2542
Buchdunger E, Cioffi CL, Law N et al (2000) Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 295:139–145
Blanke CD, Rankin C, Demetri GD et al (2008) Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26:626–632
Cohen MH, Cortazar P, Justice R, Pazdur R (2010) Approval summary: imatinib mesylate in the adjuvant treatment of malignant gastrointestinal stromal tumors. Oncologist 15:300–307
Joensuu H (2008) Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39:1411–1419
Rutkowski P, Bylina E, Wozniak A et al (2011) Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour: the impact of tumour rupture on patient outcomes. Eur J Surg Oncol 37:890–896
Fletcher CD, Berman JJ, Corless C et al (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33:459–465
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130:1466–1478
Rutkowski P, Nowecki ZI, Michej W et al (2007) Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol 14:2018–2027
Takahashi T, Nakajima K, Nishitani A et al (2007) An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol 12:369–374
Hohenberger P, Ronellenfitsch U, Oladeji O et al (2010) Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 97:1854–1859
Rossi S, Miceli R, Messerini L et al (2011) Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol 35:1646–1656
DeMatteo RP, Ballman KV, Antonescu CR et al (2009) Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373:1097–1104
DeMatteo RP, Owzar K, Antonescu CR et al (2008) Efficacy of adjuvant imatinib mesylate following complete resection of localized, primary gastrointestinal stromal tumor (GIST) at high risk of recurrence: the US Intergroup phase II trial ACOSOG Z9000. ASCO 2008 Gastrointestinal Cancers Symposium (Abstract 8)
European Medicines Agency (2010) Glivec. EMA website. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000406/human_med_000808.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124. Accessed 6 Feb 2013
Li J, Gong JF, Wu AW, Shen L (2011) Post-operative imatinib in patients with intermediate or high risk gastrointestinal stromal tumor. Eur J Surg Oncol 37:319–324
Kang B Sr, Lee J, Ryu M et al (2009) A phase II study of imatinib mesylate as adjuvant treatment for curatively resected high-risk localized gastrointestinal stromal tumors. J Clin Oncol (Meeting Abstracts) 27:e21515
Joensuu H, Eriksson M, Sundby HK et al (2012) One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 307:1265–1272
Casali PG, Le Cesne A, Velasco AP et al (2013) Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): The EORTC/AGITG/FSG/GEIS/ISG randomized controlled Phase III trial. J Clin Oncol (Meeting Abstracts) 31:10500
ClinicalTrials.gov (2012) Five year adjuvant imatinib mesylate (Gleevec®) in gastrointestinal stromal tumor (GIST). ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT00867113. Accessed 6 Feb 2013
Jiang WZ, Guan GX, Lu HS et al (2011) Adjuvant imatinib treatment after R0 resection for patients with high-risk gastrointestinal stromal tumors: a median follow-up of 44 months. J Surg Oncol 104:760–764
Corless CL, Ballman KV, Antonescu C et al (2010) Relation of tumor pathologic and molecular features to outcome after surgical resection of localized primary gastrointestinal stromal tumor (GIST): results of the intergroup phase III trial ACOSOG Z9001. J Clin Oncol (Meeting Abstracts) 28:10006
Lasota J, vel Dobosz AJ, Wasag B et al (2007) Presence of homozygous KIT exon 11 mutations is strongly associated with malignant clinical behavior in gastrointestinal stromal tumors. Lab Invest 87:1029–1041
Corless CL, Heinrich MC (2008) Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 3:557–586
Heinrich MC, Corless CL, Demetri GD et al (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21:4342–4349
Debiec-Rychter M, Sciot R, Le Cesne A et al (2006) KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42:1093–1103
Corless CL, Schroeder A, Griffith D et al (2005) PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 23:5357–5364
Weisberg E, Wright RD, Jiang J et al (2006) Effects of PKC412, nilotinib, and imatinib against GIST-associated PDGFRA mutants with differential imatinib sensitivity. Gastroenterology 131:1734–1742
Cassier P, Fumagalli E, Rutkowski P et al (2012) Outcome of patients with platelet derived growth factor receptor alpha-mutated GIST in the tyrosine kinase inhibitor era. Clin Cancer Res 18:4458–4464
Lamba G, Gupta R, Lee B, Ambrale S, Liu D (2012) Current management and prognostic features for gastrointestinal stromal tumor (GIST). Exp Hematol Oncol 1:14
Joensuu H, Hohenberger P, Corless CL (2013) Gastrointestinal stromal tumour. Lancet [Epub ahead of print]
Blay JY, Le Cesne A, Ray-Coquard I et al (2007) Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 25:1107–1113
Le Cesne A, Ray-Coquard I, Bui BN et al (2010) Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol 11:942–949
Ray-Coquard IL, Bin Bui N, Adenis A et al (2010) Risk of relapse with imatinib (IM) discontinuation at 5 years in advanced GIST patients: results of the prospective BFR14 randomized phase III study comparing interruption versus continuation of IM at 5 years of treatment: A French Sarcoma Group Study. J Clin Oncol (Meeting Abstracts) 28:10032
Le Cesne A, Ray-Coquard IL, Bui Nguyen B et al (2011) Time to secondary resistance (TSR) after interruption of imatinib (IM) in advanced GIST: updated results of the prospective French Sarcoma Group randomized phase III trial on long-term survival. J Clin Oncol 29:10015
Domont J, Blay J, Ray-Coquard IL et al (2011) Influence of imatinib interruption and imatinib rechallenge on the residual tumor volume in patients with advanced GIST: result of the BFR14 prospective French Sarcoma Group randomized phase III trial. J Clin Oncol 29:10054
Reichardt P, Hartmann J, Sundby Hall K et al (2011) Response to imatinib rechallenge of GIST that recurs following completion of adjuvant imatinib treatment: the first analysis in the SSGXVIII/AIO trial patient population. Eur J Cancer 45:31LBA
Le Cesne A, Ray-Coquard I, Bin Bui N et al (2010) Time to onset of progression after imatinib interruption and outcome of patients with advanced GIST: results of the BFR14 prospective French Sarcoma Group randomized phase III trial. J Clin Oncol (Meeting Abstracts) 28:10033
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Jared Wels, PhD, for his medical editorial assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reichardt, P., Joensuu, H. & Blay, JY. New fronts in the adjuvant treatment of GIST. Cancer Chemother Pharmacol 72, 715–723 (2013). https://doi.org/10.1007/s00280-013-2248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2248-0